

The research infrastructure at Dr. D Y Patil Medical College, Hospital and Research Centre, Pune includes the OPDs and the In-patient Wards catering to the patients attending the hospital, the staff and employees taking care of the patients in the hospital as part of the 37 Departments at the Medical College, the Clinical Trials Unit, the Central Research Facility and the Central Clinical Laboratory. Facilities are provided for initiating new intramural research projects at the medical college by providing seed money. Facilities are also provided for patenting new products and for copyrights by providing the guidance and the funds required for the patents and the copyrights. The infrastructure also includes the excellent library on the campus and the e-resources available on the college website at medical.dpu.edu.in.
Dr. D.Y Patil Medical College, Hospital and Research Centre acknowledges the articles published and recognized in the following journals which has a universal recognition in medical science:
To help the faculty and the students with their research related activities so that their articles are published in the above journals, several e-resources have been provided. These include access to :
Scopus is an expertly curated abstract and citation database with powerful search capabilities, global content, research metrics, and structured data, that support the entire research ecosystem. With Scopus, one can quickly find relevant and authoritative research manuscripts, identify experts and gain access to reliable data, metrics, and analytical tools.
Unique Value Delivered by Scopus
Scopus is used to:
Web of Science is a comprehensive and widely used research database and citation indexing platform that plays a crucial role in supporting researchers throughout the process of publishing their research articles. Web of Science is an indispensable resource for researchers at various stages of the publication process.
It assists in finding relevant literature, searching for publications of authors, searching for publications on a research topic, assessing the impact of research, connecting with peers, selecting good journals, and staying informed about the latest developments in the research field. These capabilities ultimately contribute to the successful publication of research articles and the advancement of knowledge in the academic community. Researchers can harness the power of Web of Science to enhance their research and ensure that their work reaches a broader and more influential audience.
Research to Publication is a research methodology and publishing programme specifically designed for doctors and healthcare researchers, brought to students and faculty members by BMJ in collaboration with the University of California, San Francisco (UCSF).
There are 8 multimedia courses focused entirely on healthcare research. BMJ’s research editors and UCSF’s academics guide learners through the entire process, from designing a study to seeing it published in an international journal.
Interactive self-study modules give learners the skills necessary to conduct investigations and overcome the challenges associated with getting published in international journals.
As a student or health professional, one has spent many hours in literature searching and referencing for research. Faculty members may have to reject protocols or publications that had old or irrelevant references or citations in the wrong style.
The root cause for this is that medical students are not taught the structured methods of literature searching and referencing in the medical curriculum.
QMed Knowledge Foundation has hosted courses that help anyone—from UG students upwards—learn these skills. Dr. D. Y. Patil Medical College, Hospital & Research Centre has subscribed to these for all students and faculty members of the institution.
These e-Learning courses can be done at one’s own pace, viewing recorded videos at convenience. "Learn in bytes"—as much as one can in a day. As one learns a specific skill, one earns a badge for that skill. After completing the MCQs, one can download a certificate upon passing the final assessment, which can be retaken multiple times to achieve the highest score.
One can save hours in searching and referencing, using the time saved for actual research, thus reducing or eliminating the possibility of rejection of publications.
UpToDate provides concise updated information on various clinical topics, calculates various health parameters, and gives information on drug-drug interactions and various pathways. All clinical and drug-related information can be accessed on mobile phones, making it useful for patient management and for accessing clinical information while conducting or writing research projects.
Cochrane Interactive Learning (CIL) is a modular, self-directed educational tool for researchers performing systematic reviews. With the exponential growth of published and unpublished biomedical evidence, systematic reviews are critical tools for informing clinical practice and decision-making.
Both new and experienced researchers must adhere to rigorous standards to produce unbiased and transparent systematic reviews. CIL provides an accessible, step-by-step guide for navigating the systematic review process.
Developed by the Cochrane Learning and Support Department in collaboration with multiple e-learning partners, all modules align with the Cochrane Handbook for Systematic Reviews of Interventions.
Completion of all nine modules takes more than ten hours on average, and content is regularly updated. The modules feature a filtering tool for sorting content by status or topic, a progress tracking bar, and “Cochrane Tips” for additional guidance.
At the end of each module, there is a self-assessment including multiple-choice and fill-in-the-blank questions. Users receive two attempts to answer each question correctly before being directed back for further learning. A personalized certificate is available for download upon successful completion of each assessment.
Students and faculty at Dr. D. Y. Patil Medical College, Hospital and Research Centre have access to publish in the indexed journals of BMJ, Karger, and the journal Cureus. In the Karger subscription, there are 102 journals available for publication. In the BMJ subscriptions, 64 journals are available for postgraduate and faculty publication.
Faculty and postgraduates have been involved in research projects and publishing in Scopus, WOS, and PubMed indexed journals. The number of publications from 2014 to 2024 is provided in the graphs below:
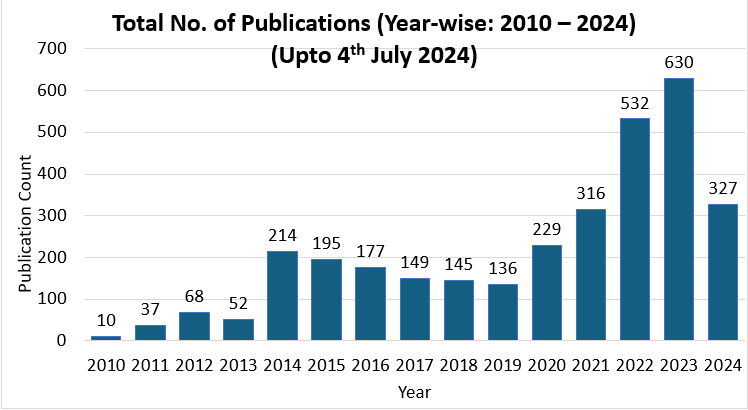
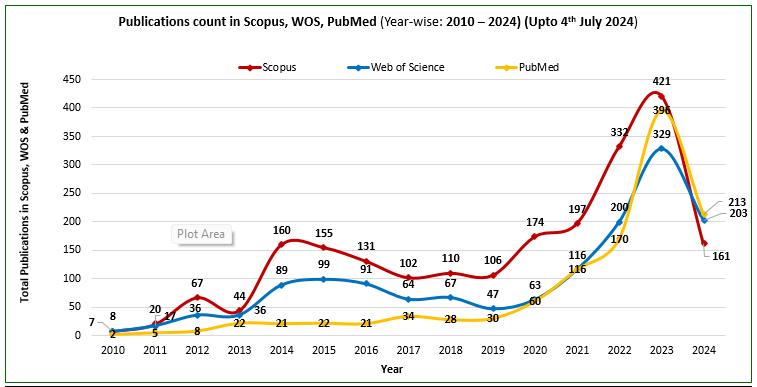
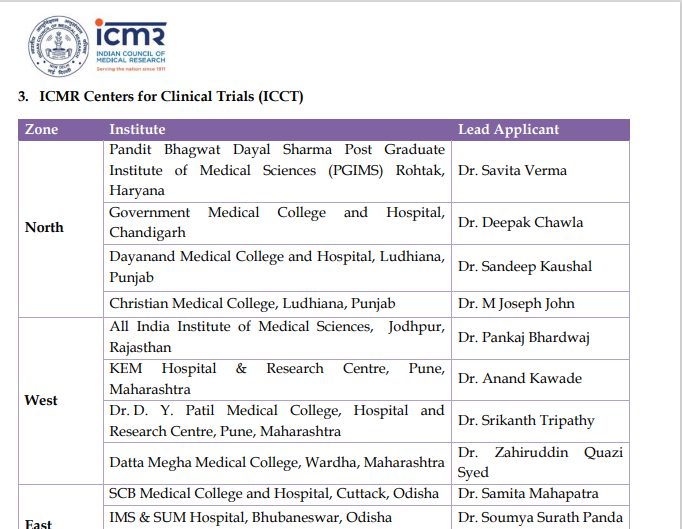
Dr D Y Patil Medical College, Hospital and Research Centre, Pune has been recognized by the Indian Council of Medical Research as the ICMR Centre for Clinical Trials for the Western Zone in India. Further details are given in the communication from ICMR received below:

Funding Agency: Indian Council of Medical Research (ICMR), Delhi
Principal Investigator: Dr. Sachin Shivnitwar
Project Duration: 14 June 2021 to 31 August 2024 (ongoing)
Budget Sanctioned: ₹34,05,192.77
Total Patients Enrolled: 669
Gender Distribution:
Age Distribution:
Primary Objective: Establish a digital repository for comprehensive data collection on clinical and laboratory aspects, treatments, and outcomes of COVID-19 patients hospitalized in India.
Secondary Objectives:
The study commenced after securing approvals from the Central Ethics Committee and Institutional Ethics Committee. It involves prospective data collection on all COVID-19-confirmed hospitalized patients. Verbal autopsy forms are available for post-discharge deaths, with telephonic interviews conducted.
Funding Agency: ICMR - National Institute of Epidemiology, Chennai
Principal Investigator: Dr. Sachin Shivnitwar
Project Duration: October 2021 to March 2023
Budget Sanctioned: ₹1,50,000
Enrollment: 5 cases with 25 controls
This study investigates sudden deaths in individuals aged 18-45, comparing data from those who died suddenly with that of living individuals. Factors such as COVID history, vaccination status, and physical activity before the event are explored.
The study will be conducted in selected hospitals and community levels, utilizing existing case records and family interviews for data collection.
Funding Agency: ICMR - National Institute of Epidemiology, Chennai
Principal Investigator: Dr. Sachin Shivnitwar
Project Duration: October 2021 to December 2022
Budget Sanctioned: ₹1,68,700
Enrollment: 70 cases with 280 controls
This study investigates potential severe health issues linked to COVID-19 vaccines among individuals aged 18-45. It evaluates thrombotic events following vaccination to ensure safety and maintain public confidence.
The study employs a matched case-control design, collecting data from hospitals on thrombotic events and matching controls based on hospitalization dates.
The list below contains the details of the clinical trials that were carried out at the Dr D Y Patil Medical College, Hospital and Research Centre during the period from 2022 to 2023.
| Sr No | Project Proposal Title | PI of Study | Department | Sponsor | Start | Completed | Budget |
|---|---|---|---|---|---|---|---|
| 1 | “Prevalence of microalbuminuria as a marker of early nephropathy in obese non-DM and non-hypertensive patients with reference to adipokine levels” | Dr. Varsha Bhatt | Medicine & clinical trial / vaccine trial unit | ICMR | 2019 | Completed in 2022 | 15,17,513/- |
| 2 | “A phase 2/3, observer-blind, randomized, controlled study to determine the safety and immunogenicity of COVOVAX [SARS-CoV-2 recombinant spike protein nanoparticle vaccine (SARS-CoV-2 rS) with Matrix-M1™ adjuvant] in Indian adults” | Dr. Varsha Bhatt | Medicine & clinical trial / vaccine trial unit | Serum Institute of India Private Limited (SIPL) and ICMR (Co-sponsor) | Mar 2021 | Completed in MAY 22 | 35,56,662/- |
| 3 | Randomized, Phase I/II, Placebo-controlled, Dose-Ranging, study to evaluate the Safety, Tolerability and Immunogenicity of the candidate HGCO19 (COVID-19 vaccine) in healthy adult subjects | Dr. Prakash Shende | Medicine & clinical trial / vaccine trial unit | Gennova Biopharmaceuticals Limited | May 2021 | Completed in 2022 | 81,716/- |
| 4 | A Prospective, Multicentre, Randomized, Active-controlled, Observer-blind, Phase II study seamlessly followed by a Phase III study to evaluate the Safety, Tolerability and Immunogenicity of the candidate HGCO19 (COVID-19 vaccine) in healthy subjects | Dr. Prakash Shende | Medicine & Clinical trial / vaccine Trial unit | Gennova Biopharmaceuticals Limited | 21 Sep 2021 | Completed in 2023 | 36,68,057/- |
| 5 | ICMR-WHO Solidarity Trial Plus: - “An international randomized trial of additional treatments for COVID-19 in hospitalized patients | Dr. Shubhangi Kanitkar | Medicine & Clinical trial / vaccine Trial unit | ICMR and WHO | Oct 2021 | Completed in 2023 | 4,57,269/- |
| 6 | “National Clinical Registry for COVID-19” | Dr. Dr. Sachin K. Shivnitwar | Medicine & Clinical trial / vaccine Trial unit | ICMR | 2021 | ONGOING | 34,05,192 |
| 7 | RANDOMISED EVALUATION OF COVID-19 THERAPY (RECOVERY) | Dr. Shubhangi Kanitkar | Medicine & Clinical trial / vaccine Trial unit | University of Oxford, UK India Co-Ordinator- ICMR | 14 May 2022 | Completed in 2023 | 2,97,997/- |
| 8 | A phase 3, observer-blind, randomized, controlled study to evaluate the safety and immunogenicity of a booster dose of Covovax in Indian adults who have received primary vaccination against COVID-19 | Dr. Srikanth Tripathy | Clinical trial / vaccine Trial unit | Serum Institute of India Private Limited (SIPL) and ICMR (Co-sponsor) | May 2022 | Completed in May 2023 | 11,70,998/- |
| 9 | A prospective, multi-center, single arm, open label, study to evaluate safety and efficacy of RanizuRelTM containing Ranibizumab for intra-vitreal injection, manufactured by Reliance Life Sciences, Pvt. Ltd. India in patients with neovascular (wet) age-related macular degeneration. | Dr. Nilesh Giri | Ophthalmology & Clinical trial / vaccine Trial unit | Reliance Life Sciences Pvt. Ltd | Sep 2022 | ONGOING | 11,63,600/- |
| 10 | A Randomized, Double-Blind, Vehicle-Controlled, Three Ann, Parallel Group, Multi-Centric, Clinical Study to Establish the Therapeutic Equivalence of Tacrolimus 0.1% Topical Ointment of Intas Pharmaceuticals Limited with Protopic® 0.1% Topical Ointment in Adult Patients with Moderate to Severe Atopic Dermatitis | Dr. Hemant Talnikar | Dermatology & Clinical trial / vaccine Trial unit | Intas Pharmaceuticals Limited CRO- Lambda Therapeutic research | Oct 2022 | Completed. Close out pending | 3,07,727/- |
| 11 | A Randomized, Double-Blind, Vehicle-Controlled, Three Ann, Parallel Group, Multi-Centric, Clinical Study to Establish the Therapeutic Equivalence of Tacrolimus 0.3% Topical Ointment of Intas Pharmaceuticals Limited with Protopic® 0.3% Topical Ointment in Adult Patients with Moderate to Severe Atopic Dermatitis | Dr. Hemant Talnikar | Dermatology & Clinical trial / vaccine Trial unit | Intas Pharmaceuticals Limited CRO- Lambda Therapeutic research | Oct 2022 | Completed. Close out pending | 1,95,993/- |
| 12 | LRP/LUBT010/2016/008: A Global, Phase III, Double Blind, Randomized Controlled Study to Compare the Efficacy, Safety & Immunogenicity of LUBT010 with Lucentis® in Patients with Neovascular Age-Related Macular Degeneration | Dr. Nilesh Giri | Ophthalmology & Clinical trial / vaccine Trial unit | LUPIN pharmaceuticals Pvt. Ltd CRO- IQVIA | Oct 2022 | ONGOING | 10,76,454/- |
| 13 | A Prospective, Multi-centre, Open-labelled, Randomized, Phase II study seamlessly followed by a Phase III study to evaluate the Safety, Tolerability and Immunogenicity of GEMCOVAC-OM as a booster in Subjects 18 years of age and older. | Dr. Vikram Vikhe | Medicine & Clinical trial / vaccine Trial unit | Gennova Biopharmaceuticals Limited CRO-JSS Medical Research Asia Pacific Pvt. Ltd. | Nov 2022 | Completed. Close out pending | 64,53,000/- |
| 14 | A prospective, post marketing clinical follow up study to evaluate the safety and Performance of Sirolimus Eluting Cobalt Chromium Coronary Stent System in Real World Indian population. | Dr. Susheel Kumar Malani | Department of Cardiology & Clinical trial / vaccine Trial unit | Translumina Therapeutics LLP | Nov 2022 | ONGOING | 1,29,625 |
| 15 | A Phase III, Prospective, Randomized, Parallel group, Double-blind, Multicentre Study to Compare the Efficacy, Safety, and Immunogenicity of Lupin’s Aflibercept with Eylea® in Patients with Neovascular Age-Related Macular Degeneration | Dr. Nilesh Giri | Ophthalmology & Clinical trial / vaccine Trial unit | LUPIN pharmaceuticals Pvt. Ltd | Apr 2023 | ONGOING | 13,47,005/- |
| 16 | Factors associated with sudden deaths among adults aged 18–45 years, India: Multicentric matched case-control study | Dr. Sachin Shivnitwar | Medicine & Medical Research | ICMR | May 2023 | ONGOING | 1,50,000 |
| 17 | Effect of COVID-19 vaccine on thrombotic events among 18–45-year-old population in India, 2022: Multicentric hospital-based matched Case control study | Dr. Sachin Shivnitwar | Medicine & Medical Research | ICMR | May 2023 | ONGOING | 1,68,700 |
| 18 | A Prospective, Multi-centre, Single-arm, Phase IV Study to Assess the Safety and Efficacy of Fixed Dose Combination of Brinzolamide and Timolol Ophthalmic Suspension to Decrease Intraocular Pressure (IOP) in Adult Patients with Open-angle Glaucoma or Ocular Hypertension for whom Monotherapy Provides Insufficient IOP Reduction." ICR/22/002 | Dr. Megha Ramnik Kotecha | Ophthalmology & Clinical trial / vaccine Trial unit | SUN PHARMA | May-23 | ONGOING | 4,59,084 |
| 19 | Prospective, multi-center, randomized, open label, active control, phase 2 clinical study to evaluate immunogenicity, safety and tolerability of single heterologous booster dose of RelCoVax® (Protein Subunit Vaccine of Reliance Life Sciences against SARS-CoV-2 Virus) with Corbevax® (Protein Subunit Vaccine of Biological E Ltd. against SARS-CoV-2 Virus) | Dr. Srikanth Tripathy | Clinical trial / Vaccine Trial unit | Reliance Life Sciences Pvt. Ltd | 11-Oct-23 | ONGOING | 9,10,960 |
| 20 | A Prospective, multi-center, open label, single-arm, phase I study to evaluate the safety of GBL1204 in patients with neovascular age-related macular degeneration. | Dr. Nilesh Giri | Ophthalmology & Clinical trial / vaccine Trial unit | Gennova Biopharmaceuticals Limited | 06 Mar 2024 | ONGOING | 500000 |
| 21 | A prospective, randomized, single blind, parallel, active controlled, multicenter, non-inferiority phase iii study to evaluate the immunogenicity and safety of live attenuated varicella vaccine of Sinovac biotech ltd, China compared to variped® vaccine (varicella vaccine live i.p by MSD pharmaceuticals pvt ltd, India) in healthy pediatric subjects in India. | Dr. Shailaja Mane & Dr. Srikanth Tripathy | Pediatric Department & Clinical trial / vaccine Trial unit | Clinical Research Network India, (CRO) | 19 Jun 2024 | ONGOING | |
| 22 | A Multicenter, Randomized, Observer-blind, Non-inferiority, Phase III Study to Evaluate the Immunogenicity and Safety of BoostagenREDTM (combined tetanus toxoid, reduced diphtheria toxoid, reduced recombinant pertussis vaccine) by BioNet-Asia compared to ADACEL® vaccine by Sanofi Pasteur Limited in Healthy Subjects Aged 4-65 years. | Dr. Srikanth Tripathy & Dr. Shailaja Mane | Clinical trial / vaccine Trial unit | Clinical Research Network India, (CRO) | 18 Jun 2024 | ONGOING | |
| 23 | A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy, Immunogenicity and Safety of Single dose of Dengue Tetravalent Vaccine, Live Attenuated (Recombinant, Lyophilized) – “DengiAll” of Panacea Biotec Limited in Healthy Indian Adults | Dr. Shubhangi Kanitkar | Medicine & Clinical trial / vaccine Trial unit | ICMR & Panacea Biotec Limited | Will start in Jul 2024 | Jul 2024 | |
| 24 | A Phase 3, Multicenter, Open-label, Rater-blind, Active-controlled Study to Evaluate the Efficacy and Safety of Bedaquiline for the Treatment of Multibacillary Leprosy When Combined With Rifampicin and Clofazimine [LIGHT] | Dr. Ayush Gupta | Dermatology & Clinical trial / vaccine Trial unit | Johnson & Johnson Innovative Medicine | All approval received will start in Jul 2024 | Jul 2024 | |
| 25 | A prospective, interventional, randomized, comparative open label pivotal clinical study to assess the efficacy of Serioss® as bone void filler/bone substitute. | Dr. Rahul Salunkhe | Orthopaedics Department & Clinical trial / vaccine Trial unit | Serigen Mediproducts Pvt Ltd. | Site selected, Documents will submit to EC. | ||
| 26 | “Ultrasound-guided radiofrequency ablation of uterine fibroids with the GYIDE system” short-term evaluation of effect of treatment on quality of life and symptom severity. | Dr. Meenal Patvekar | OBGY, DPU Cell, & Clinical trial / vaccine Trial unit | BioAgile Therapeutics Pvt. Ltd. (CRO) | Site selected, Documents will submit to EC. | ||
| 27 | A Prospective, Randomized, Active Controlled, Double-blind, Multicenter Phase III Clinical Study to Evaluate the Immunogenicity and Safety of 23-Valent Pneumococcal Polysaccharide Vaccine in Healthy Adults. | Dr. Srikanth Tripathy | Clinical trial / vaccine Trial unit | Clinical Research Network India, (CRO) | Site selected, Documents will submit to EC. | ||
| 28 | A Randomized, Multicenter, Open Label, Comparative Study to Evaluate the Immunogenicity and Reactogenicity of a Fully Liquid Pentavalent DTwP-HepB-Hib Vaccine [MyFive™] of Panacea Biotec Ltd. With Pentavalent DTwP-HepB-Hib Vaccine [Pentanal SD®] of Serum Institute of India Ltd. following Primary immunization in Healthy Infants. | Dr. Shailaja Mane | Pediatric Department & Clinical trial / vaccine Trial unit | Panacea Biotec Limited (Sponsor) & Clinical Research Network India, (CRO) | Site selected, Documents will submit to EC. | ||
| 29 | A Prospective, Randomized, Observer Blinded, Parallel, Active Controlled, Multicentric, Non-Inferiority Phase III Study to Evaluate The Immunogenicity And Safety Of Group ACYW135 Meningococcal Conjugate Vaccine in Comparison To a Marketed Meningococcal Vaccine In Healthy Indian Subjects. | Dr. Shailaja Mane & Dr. Tripathy | Pediatric Department & Clinical trial / vaccine Trial unit | Panacea Biotec Limited (Sponsor) & Clinical Research Network India, (CRO) | Site selected, Documents will submit to EC. | ||
| 30 | A Randomized, Open label, Multicenter, Phase II / III study to assess and compare the immunogenicity and safety of NUCOVAC® -11 {Pneumococcal Polysaccharide conjugate vaccine (Adsorbed), 11 valent}of Panacea Biotec Ltd. with PREVENAR 13® of Pfizer Inc. in healthy infants (3+1 immunization schedule). | Dr. Shailaja Mane & Dr. Tripathy | Pediatric Department & Clinical trial / vaccine Trial unit | Panacea Biotec Limited (Sponsor) & Clinical Research Network India, (CRO) | Site selected, Documents will be submitted to IEC. | ||
| 31 | A Phase 3, Randomized, Double-blind, Controlled, Multi-Center Study to Compare Immunogenicity and Safety of SIIPL Meningococcal ACYWX Conjugate Vaccine (NmCV-5) with that of Licensed Meningococcal ACWY Vaccine Menactra® in Healthy Indian Children of 9 months to 17 Years of Age | Dr. Shailaja Mane & Dr. Tripathy | Pediatric Department & Clinical trial / vaccine Trial unit | Serum Institute of India Private Limited (SIPL) | Site selected | ||
| 32 | Phase 3 Multi-Center, Randomized, Double-Blind, Study to Assess the Efficacy and Safety of Treatment with bepirovirsen in Nucleos(t)ide Analogue treated Participants with Chronic Hepatitis B Virus (B-Well). | Dr. Abhijit Karad | Gastroenterology Department & Clinical trial / vaccine Trial unit | GSK | Site selected, Documents will submit to EC. | ||
| 33 | A Phase III, Multicentre, Randomized, Observer -blind, Active-controlled, Parallel group, Non-inferiority Study to Evaluate Immunogenicity and Safety of Varicella vaccine (BARYCELA) in Healthy Pediatric Population 12 months to 12 years of age. | Dr. Shailaja Mane & Dr. Tripathy | Pediatric Department & Clinical trial / vaccine Trial unit | Dr. Reddy’s Laboratories Ltd & CRO | Site selected |
Among the extramural projects funded from within the country, there are 19 research project proposals that are either completed recently (11 projects) or are ongoing (8 projects) at our medical college, another seven projects are on the verge of being initiated soon and nine more research projects in the pipeline.
MOU for research collaboration has been signed between DPU Dr. DY Patil Medical College, Hospital and Research Centre, Pune and HaystackAnalytics Pvt. Ltd. (IIT Mumbai based start up company). They mainly deal with Whole Genome Sequencing of TB strains (both susceptible and resistant) and have also developed methods for the diagnosis of sepsis.
Among the international collaborative research studies of our medical college, presently we have two NIH funded collaborative projects worth $8,00,560/ and one CDC funded study worth $ 1,77,053/ USD in collaboration with Johns Hopkins university. USA
We have signed an MOU for launching a NIH funded study worth $ 1,80,958/ with Boston University, USA and the project is ongoing.
An MOU has been signed between MRI Global & DPU for study of drug resistant TB and the grant for the $27,602/ project has been approved by MRI Global, USA and the project has been initiated.
Collaboration with Harvard University (with funding by Harvard-Dubai Centre for Global Health Delivery Amount: $66000) entitled: Assessing pathways to care among tuberculosis (TB) and drug-resistant tuberculosis (DR-TB) patients in Pune City, India: a biosocial inquiry. Study done during the period Jan 2018 to June 2019. Project has been completed.
| Year | Projects Sanctioned | Projects Completed |
|---|---|---|
| 2019-2020 | 11 | N/A |
| 2020-2021 | 15 | 3 |
| 2021-2022 | 16 | Nil |
| 2022-2023 | 9 | Nil |
| 2023-2024 | 22 | N/A |